Are you curious to know what is radial node? You have come to the right place as I am going to tell you everything about radial node in a very simple explanation. Without further discussion let’s begin to know what is radial node?
In the intricate realm of quantum mechanics, the concept of radial nodes stands as a fundamental aspect governing the behavior and structure of atomic orbitals. These nodes play a pivotal role in understanding the probability distribution of electrons within an atom, offering insights into the spatial arrangements of these subatomic particles. In this blog, we’ll explore the essence of radial nodes, their significance, and their implications in the quantum realm.
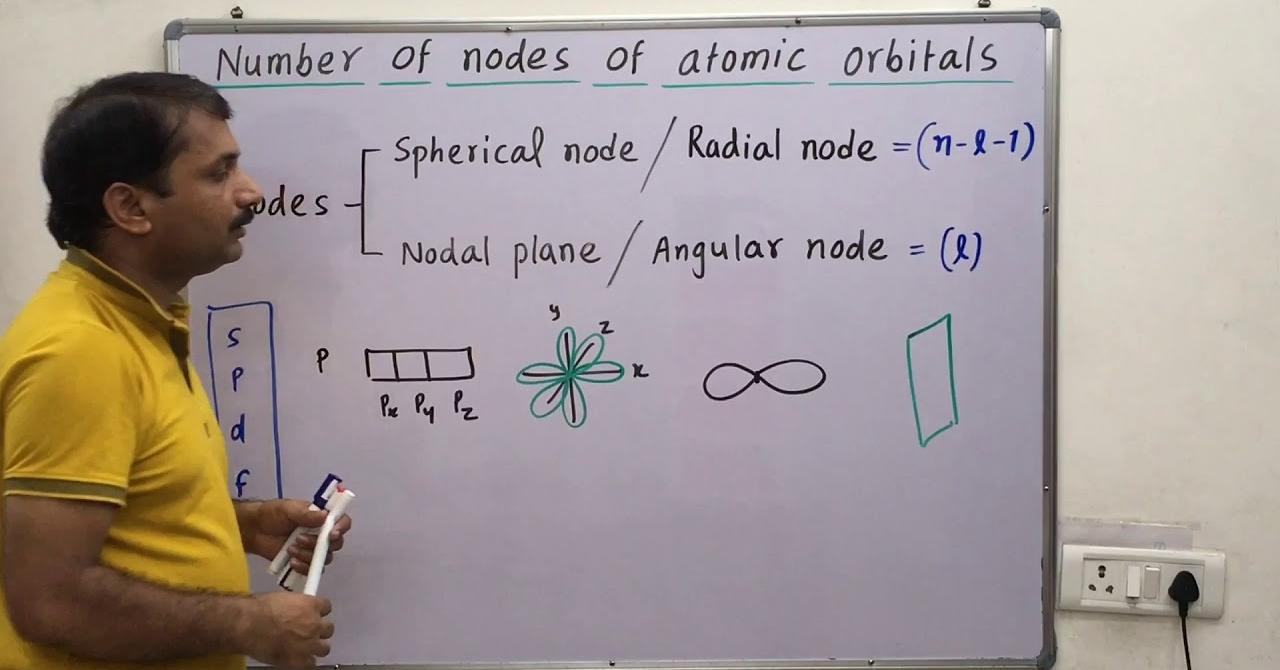
What Is Radial Node?
In quantum mechanics, the wave function describing an electron’s behavior within an atom is represented by atomic orbitals. Radial nodes are regions within these atomic orbitals where the probability of finding an electron is zero. They indicate points in space where the electron’s probability density function, which describes the likelihood of locating the electron, drops to zero along the radial direction from the nucleus.
Significance And Implications:
- Quantum State Representation: Radial nodes contribute to defining the quantum state of an electron within an atom. The number of radial nodes corresponds to specific quantum numbers and helps define the energy levels and shapes of atomic orbitals.
- Orbital Structure: The presence and distribution of radial nodes significantly influence the shapes and sizes of atomic orbitals. Nodes divide the space around the nucleus into distinct regions where the probability of electron presence varies.
- Quantum Number Relationship: The number of radial nodes within an atomic orbital is directly related to the principal quantum number (n) and the angular momentum quantum number (l). It influences the overall energy and spatial arrangement of the orbital.
Visual Representation:
- Spherical Symmetry: For s-orbitals (labeled with the quantum number l=0), there are no angular nodes but contain radial nodes that determine the spherical shape of the orbital.
- Different Angular Momenta: Orbitals with higher angular momentum (p, d, f orbitals) exhibit complex shapes due to the interplay between radial and angular nodes, influencing their three-dimensional structure.
- Quantum Mechanical Modeling: Computational models and visualizations utilize the concept of radial nodes to depict electron distribution in different atomic orbitals, aiding in understanding the spatial arrangements of electrons within atoms.
Conclusion:
Radial nodes stand as key elements in the characterization and understanding of atomic orbitals within quantum mechanics. Their existence and distribution contribute to the intricate spatial arrangement of electrons around atomic nuclei, influencing the shapes, sizes, and energy levels of these orbitals. As quantum mechanics continues to unravel the mysteries of the subatomic world, the concept of radial nodes remains an indispensable component in comprehending the behavior and organization of electrons within atoms.
FAQ
What Is Radius Node?
A radial node is a sphere (rather than an angular node which is a flat plane) that occurs when the radial wavefunction for an atomic orbital is equal to zero or changes sign .
What Is The Angular Node?
Angular node refers to a plane that passes through the nucleus. Angular nodes are usually equal to the azimuthal quantum number (l). The number of angular nodes = l The number of radial nodes = (n – l – 1) Total number of nodes = n – 1.
How Many Radial Nodes Are In 2p?
Therefore, the 2p-orbital has (2 – 2) = 0 radial nodes, as shown in the above plot. Radial nodes become evident in the higher p-orbitals ( 3p, 4p, 5p, 6p, and 7p).
What Is A Radial Node In 4s?
The 4s radial distribution function has three spherical nodes but the higher s orbitals have more. The number of nodes is related to the principal quantum number, n. In general, the ns orbital have (n – 1) radial nodes. Therefore, the 4s-orbital has (4 – 1) = 3 radial nodes, as shown in the above plot.
I Have Covered All The Following Queries And Topics In The Above Article
What Is A Radial Node
What Is Radial Node And Angular Node
What Is The Number Of Radial Node Of 3p
What Is Radial Node In Chemistry
What Is Radial Node In Chemistry
What Is Radial Node Example
What Is Radial Node Class 11
Radial Nodes Formula
Angular Nodes Formula
What Is Angular Node
Difference Between Radial And Angular Nodes
Radial Nodes In 3p
What Is Radial Node